Exciting News | JYMed’s Peptide Production Facility Receives WC Certification
Recently, JYMed’s peptide production facility, Hubei Jianxiang Biopharmaceutical Co., Ltd., received two official documents issued by the Hubei Provincial Drug Administration: the “Drug GMP Compliance Inspection Result Notification” (No. E GMP 2024-258 and No. E GMP 2024-260) and the “Export to EU Active Pharmaceutical Ingredients (API) Certificate” (WC Certificate, No. HB240039).
These documents confirm that the A102 production line in Workshop A102 (for the production of Oxytocin and Semaglutide APIs) and the A092 production line in Workshop A092 (for the production of Terlipressin API) at Hubei Jianxiang meet China’s GMP standards, which are equivalent to the EU, World Health Organization (WHO), and ICH Q7 GMP requirements for pharmaceuticals.
The inspection concluded with compliance, indicating that Hubei Jianxiang’s production quality management and regulatory practices meet domestic high standards. This development will support Hubei Jianxiang’s expansion in the global market, particularly in the EU market, enhance customer trust, foster international collaborations, and contribute to the global growth and distribution of peptide-based drugs. As international market demand increases, Hubei Jianxiang will be better positioned to meet the needs of global customers with high-quality products and services.
About JYMed
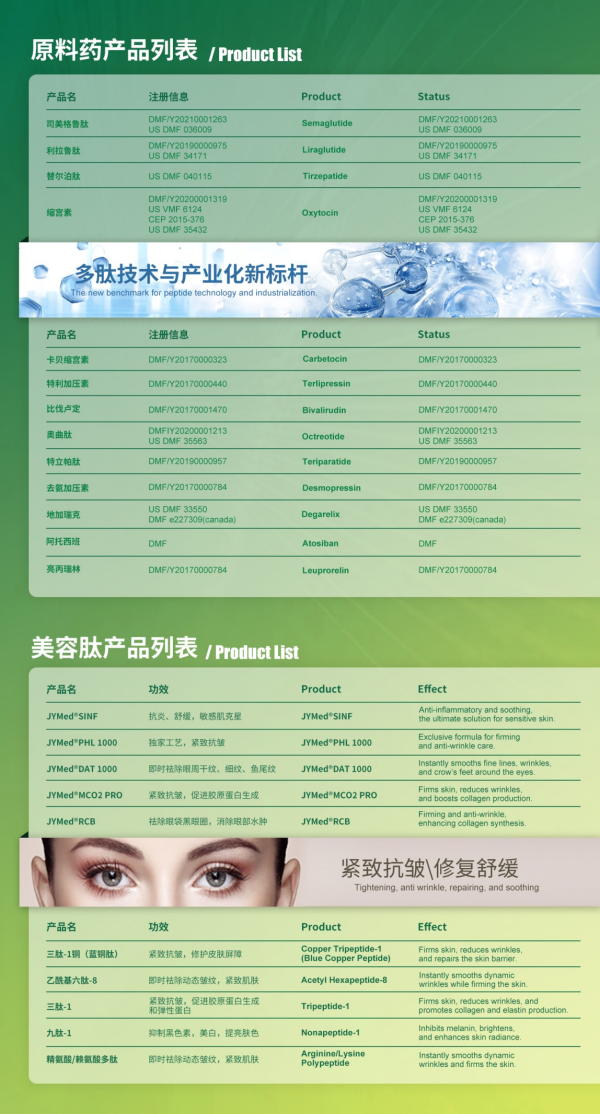
Founded in 2009, Shenzhen JYMed Technology Co., Ltd. is a biotechnology company specializing in the independent research, development, production, and sales of peptide products, alongside custom peptide R&D and manufacturing services. The company offers over 20 peptide APIs, with five products, including Semaglutide and Tirzepatide, having successfully completed U.S. FDA DMF filings.
The Hubei JX facility features 10 production lines for peptide APIs (including pilot-scale lines) that comply with cGMP standards of the U.S., EU, and China. The facility operates a comprehensive pharmaceutical quality management system and an EHS (Environmental, Health, and Safety) management system. It has passed NMPA official GMP inspections and EHS audits conducted by leading global clients.
Core Services
- Domestic and international peptide API registration
- Veterinary and cosmetic peptides
- Custom peptide synthesis, CRO, CMO, and OEM services
- PDC (Peptide Drug Conjugates), including peptide-radionuclide, peptide-small molecule, peptide-protein, and peptide-RNA conjugates
Contact Information
Address: 8th & 9th Floors, Building 1, Shenzhen Biomedical Innovation Industrial Park, Jin Hui Road 14, Kengzi Street, Pingshan District, Shenzhen, China
For International API Inquiries:
+86-755-26612112 | +86-15013529272
For Domestic Cosmetic Peptide Raw Materials:
+86-755-26612112 | +86-15013529272
For Domestic API Registration and CDMO Services:
+86-15818682250
Website: www.jymedtech.com