JYMED’S PEPTIDE PRODUCTION BASE IN HUBEI PASSES SECOND CONSECUTIVE FDA INSPECTION
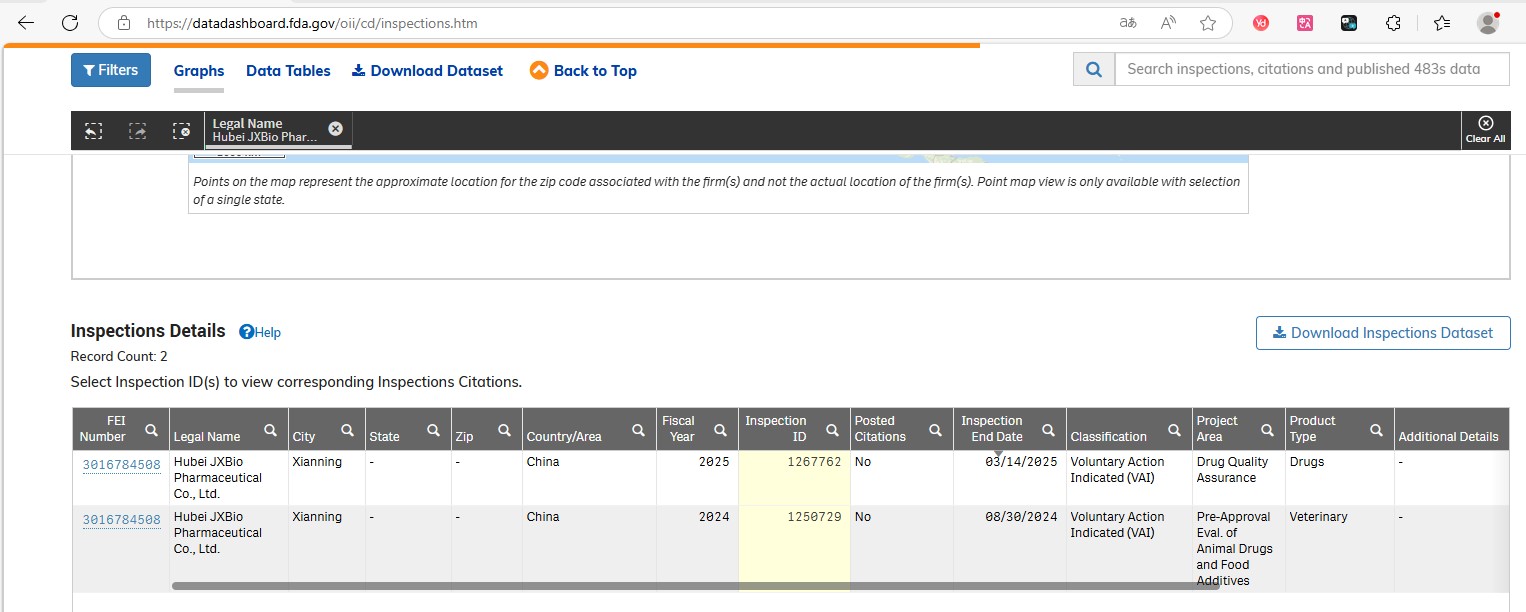
Hubei JXBio Pharmaceutical Co., Ltd., JYMed’s dedicated peptide production site, successfully completed another on-site inspection by the U.S. Food and Drug Administration (FDA) from March 10-14. The inspection, part of a Drug Quality Assurance audit, evaluated key systems including quality, production, equipment and facilities, lab controls, and material management. APIs reviewed included Liraglutide, Semaglutide, Tirzepatide, and Oxytocin.
Following the inspection, the FDA issued an official Establishment Inspection Report (EIR), confirming that Hubei JXBio’s operations meet the agency’s high standards for quality and compliance.
This marks the second consecutive FDA inspection passed by our Hubei facility, reaffirming our commitment to world-class manufacturing and quality systems. It also paves the way for smoother global product registration and strengthens our position in the international peptide market.
About JYMed
JYMed is a high-tech pharmaceutical company specializing in the research, development, production, and commercialization of peptide-based products. We also provide end-to-end CDMO services, offering tailored peptide solutions to pharmaceutical, cosmetic, and veterinary clients worldwide.
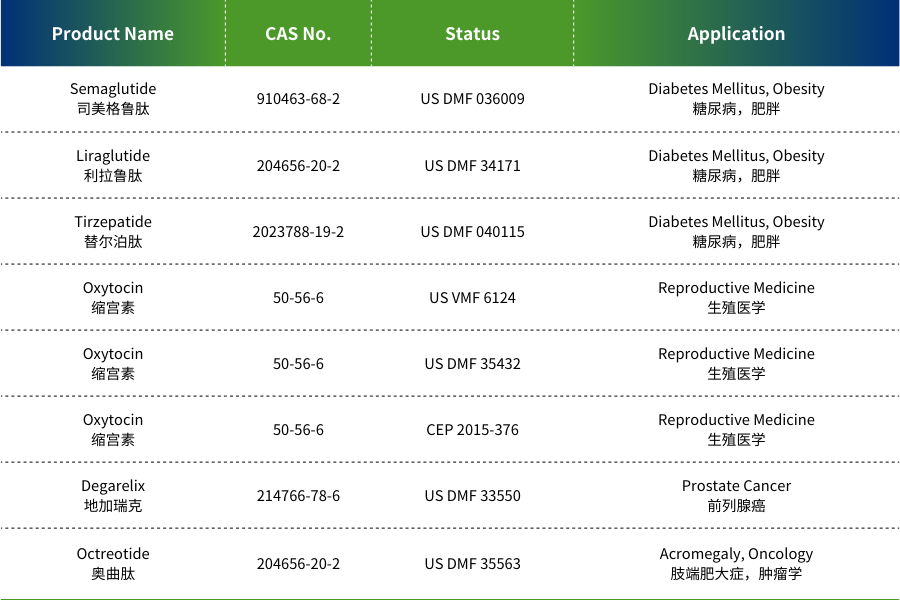
Our product portfolio includes dozens of peptide APIs. Flagship products such as Semaglutide and Terlipressin have successfully completed U.S. FDA DMF filing.
Our wholly owned subsidiary, Hubei JXBio, operates cutting-edge peptide API production lines designed to meet cGMP standards set by both the U.S. FDA and China’s NMPA. The facility includes 10 large-scale and pilot-scale production lines, supported by a rigorous pharmaceutical quality management system (QMS) and a strong environmental health and safety (EHS) program.
JXBio has passed GMP inspections by both the U.S. FDA and China’s NMPA and is recognized by leading global pharmaceutical companies for excellence in EHS management, reflecting our unwavering commitment to quality, safety, and environmental stewardship.
Core Business Areas
• Global registration and regulatory compliance for peptide APIs
• Veterinary and cosmetic peptide products
• Custom peptide development and manufacturing (CRO, CMO, OEM)
• Peptide-drug conjugates (PDCs), including:
• Peptide–radionuclide conjugates
• Peptide–small molecule conjugates
• Peptide–protein conjugates
• Peptide–RNA therapeutics
MAIN PRODUCTS
For more information about our products, please contact us:
Global API and Cosmetic Inquiries: +86-150-1352-9272
API Registration & CDMO Services (U.S. and EU markets): +86-158-1868-2250
Email: jymed@jymedtech.com
Address: Floors 8 & 9, Building 1, Shenzhen Biomedical Innovation Industrial Park,
14 Jinhui Road, Kengzi Subdistrict, Pingshan District, Shenzhen, China