- Home
- News
- Semeglutide API of Shenzhen JYMed accepted by the first batch of domestic NMPA and Registered in US FDA (DMF No. 036009) with status “A”.
Semeglutide API of Shenzhen JYMed accepted by the first batch of domestic NMPA and Registered in US FDA (DMF No. 036009) with status “A”.
In May 2022, Shenzhen JYMed Technology Co., Ltd. (hereinafter referred to as JYMed peptide) submitted an application for the registration of semaglutide API to the US Food and Drug Administration (FDA) (DMF registration number: 036009), It has passed the integrity review, and the current status is “A”. JYMed peptide has become one of the first batch of semaglutide API manufacturers in China to pass the US FDA review.
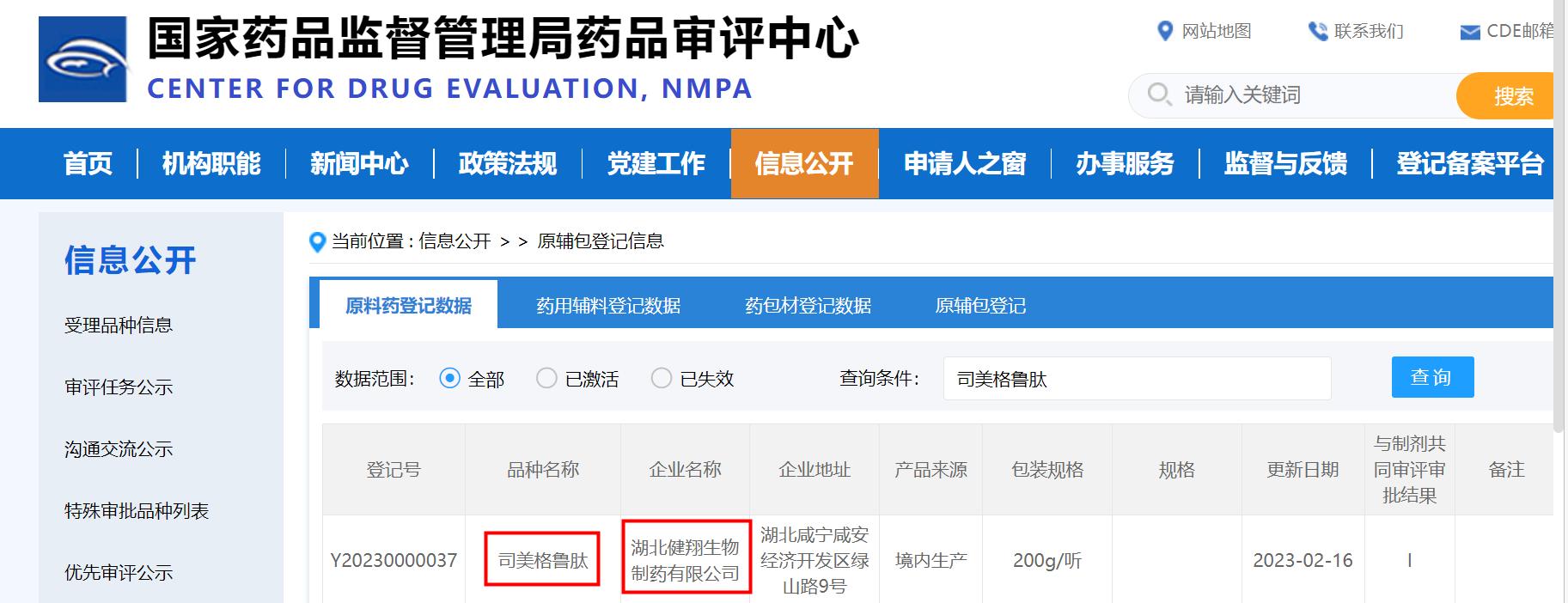
On February 16, 2023, the official website of the Drug Evaluation Center of the State Drug Administration announced that the semaglutide API [registration number: Y20230000037] registered and declared by Hubei JXBio Co., Ltd., a subsidiary of JYMed peptide, has get accepted. JYMed peptide has become one of the first raw material drug manufacturers whose marketing application for this product has been accepted in China.
About semaglutide
Semaglutide is a GLP-1 receptor agonist developed by Novo Nordisk (Novo Nordisk). The drug can increase glucose metabolism by stimulating pancreatic β cells to secrete insulin, and inhibit the secretion of glucagon from pancreatic α cells to reduce fasting and postprandial blood sugar. In addition, it reduces food intake by reducing appetite and slowing digestion in the stomach, which ultimately reduces body fat and aids in weight loss.
1. Basic information
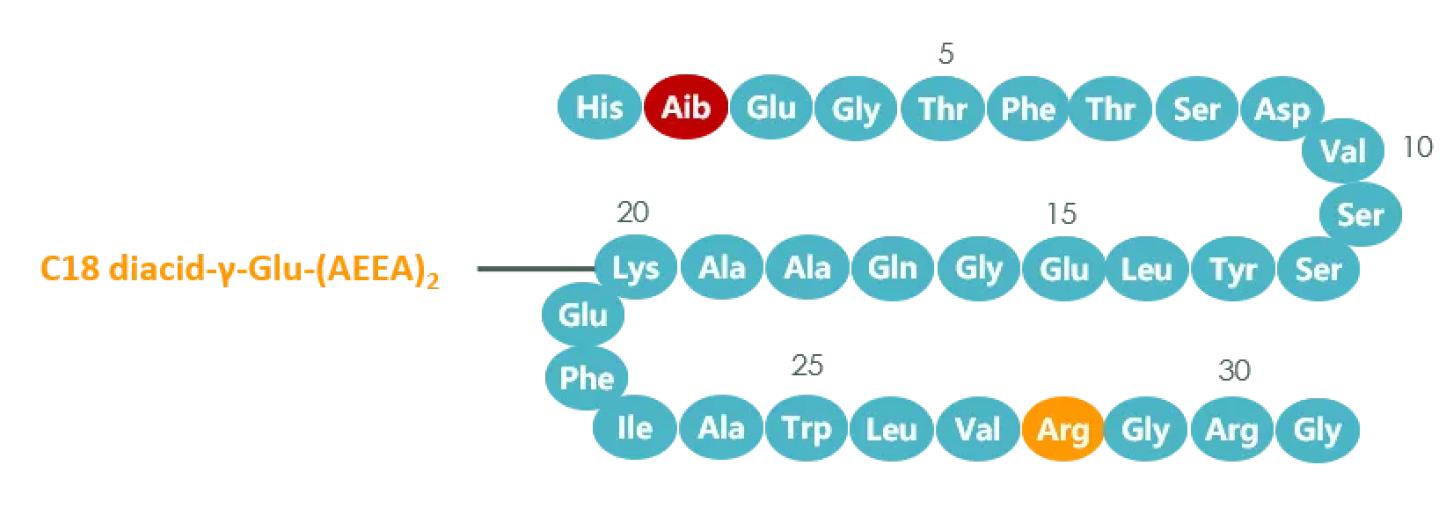
From a structural point of view, compared with liraglutide, the biggest change of semaglutide is that two AEEAs have been added to the side chain of lysine, and palmitic acid has been replaced by octadecanedioic acid. Alanine was replaced by Aib, which greatly extended the half-life of semaglutide.
Figure Structure of semaglutide
2. Indications
1) Semaglutide can reduce the risk of cardiovascular events in patients with T2D.
2) Semaglutide lowers blood sugar by stimulating insulin secretion and reducing glucagon secretion. When blood sugar is high, insulin secretion is stimulated and glucagon secretion is inhibited.
3) Novo Nordisk PIONEER clinical trial showed that oral administration of semaglutide 1mg, 0.5mg has better hypoglycemic and weight loss effects than Trulicity (dulaglutide) 1.5mg, 0.75mg.
3) Oral semaglutide is the trump card of Novo Nordisk. Oral administration once a day can get rid of the inconvenience and psychological torture caused by injection, and it is better than liraglutide (injection once a week). The hypoglycemic and weight-loss effects of mainstream drugs such as , empagliflozin (SGLT-2) and sitagliptin (DPP-4) are very attractive to patients and doctors. Compared with injection formulations, oral formulations will greatly improve the convenience of clinical application of semaglutide.
3. Summary
It is precisely because of its excellent performance in hypoglycemic, weight loss, safety and cardiovascular benefits that semaglutide has become a phenomenon-level “new star” with a huge market prospect.