- Home
- News
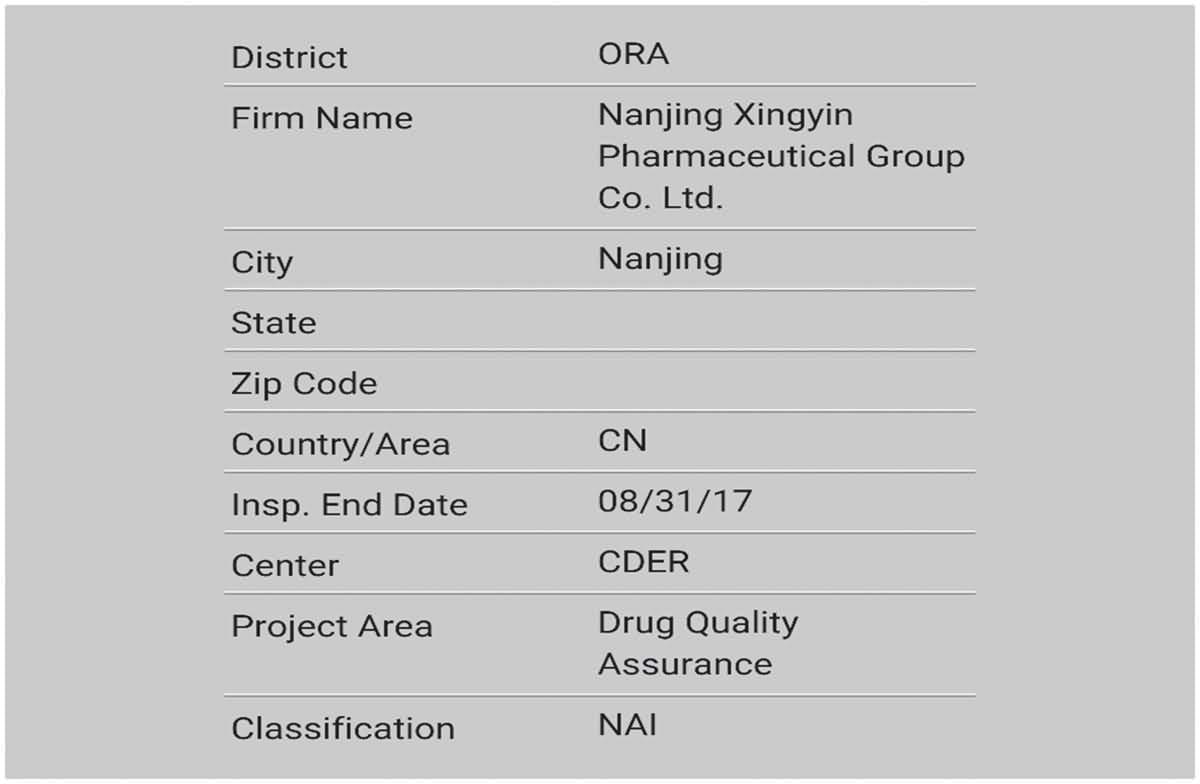
- News & EventsThe Peptide Products Division in JYMed has flawlessly passed on-site inspection from FDA.
News & EventsThe Peptide Products Division in JYMed has flawlessly passed on-site inspection from FDA.
Warmly congratulate our Polypeptide Products Division for successfully passing the US FDA on-site inspection with “zero defects”!
Passing the FDA on-site inspection with “zero defects” is a major event in our cGMP development history. It not only means that our API has obtained the passport to enter the US market, but also proves that the implementation of cGMP in our company has gradually been in line with international standards.